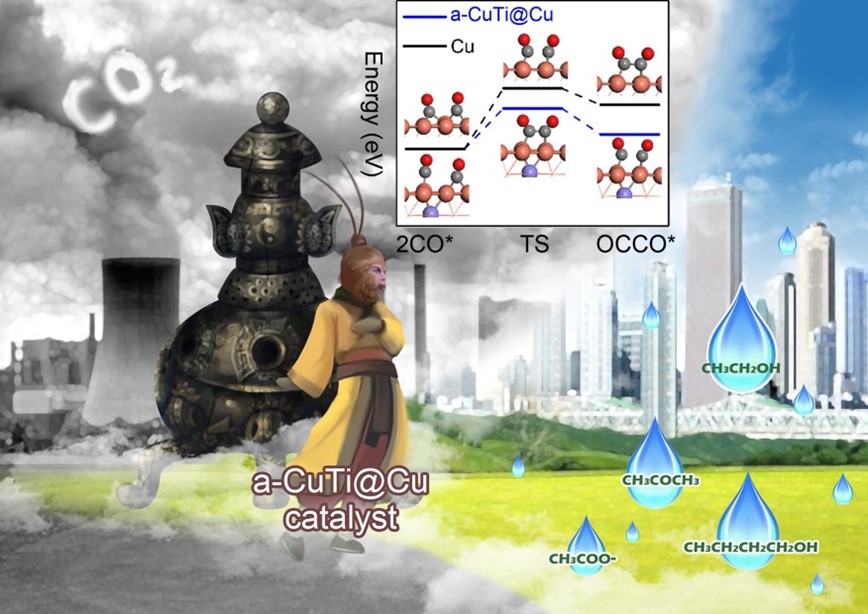
The Electrochemical reduction of CO2 to liquid fuels is highly desirable due to the high energy density and security in storage and transportation of the liquid fuels. Normally, the design of highly active and efficient catalytic materials is the key to the CO2 conversion to C2+ fuels. Among various electrocatalysts, copper (Cu)-based materials are considered the best catalyst candidates for CO2 conversion to multicarbon products. Although great efforts have been made to improve the activity and selectivity of Cu-based electrocatalysts, it remains a grand challenge for the efficient CO2 conversion to C2+ products which are mainly limited to ethylene and ethanol. Therefore, intensive research efforts have been made to improve the activity and selectivity of Cu-based electrocatalysts through different approaches. In a recent VIP paper published in Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed. 2021, 60, 26122–26127, doi.org/10.1002/anie.202110303), Hu and coworkers developed a unique Cu catalyst through an etching dealloying process. The developed Cu catalyst achieved remarkably enhanced activity, selectivity, and stability toward CO2 reduction to C2-4 liquid fuels.
In this work, the authors selected titanium (Ti), a transition metal with lower electron affinity than Cu, for combination with Cu to tailor the electron density of Cu sites. The theoretical simulation and in situ characterization in this work revealed that the electron transfer from Ti to Cu increases the electron density of coordinatively unsaturated Cu sites and thus facilitates the dimerization and trimerization of *CO intermediates, which are the key intermediates in the formation of multicarbon products via C-C coupling. Based on the characterization, the catalyst after dealloying is Cu supported on amorphous CuTi alloy (namely, a-CuTi@Cu). The a-CuTi@Cu structure is conductive and porous. Such a unique feature endows direct electron transportation, sufficient specific surface area and abundant grain boundaries, which help enhance the activity and selectivity for CO2 electroreduction simultaneously. The electrochemical CO2 reduction reaction (CO2RR) is conducted using a typical H-type cell with three electrodes in CO2-saturated 0.1M KHCO3 electrolyte. The experimental results demonstrate that the Faradaic efficiencies (FEs) for C2+ products reach the maximum (48.82%) at @0.8 V vs. RHE. The FEs for ethanol, acetone and n-butanol generation are 23.96%, 11.14% and 6.85%, respectively. Based on the comparison of CO2 reduction products at different potentials, The authors then concluded that too low potentials are insufficient to trigger the C-C coupling. To the other extreme, very high potentials would lead to the deactivation of catalysts as indicated by chronoamperometry measurements. The electron-rich feature of Cu active sites also enables the ultrahigh stability of catalyst, whose performance can be maintained for at least 3 months.
In this work, the authors developed a Cu-based catalyst which shows enhanced activity, selectivity, and stability toward electrochemical CO2 reduction to multicarbon products. The study provides an approach to tail the catalyst properties and highlights the importance of catalyst subsurface to catalytic performance, which will be beneficial to the study of CO2RR to multicarbon products. Meanwhile, further tests to evaluate the catalyst performance in a CO2 electrolyzer will be highly desirable.