Dioxide Materials' Contribution To The Hydrogen Economy
Hydrogen offers a viable solution to the world’s need for sustainable fuel for carbon-free transportation and also as a means for large-scale energy storage, especially when there is a need to balance excess renewable energy production. In order to expand our hydrogen fuel production, however, we need to develop improved alkaline water electrolyzers that are both more cost and energy efficient. There is an increased demand for new and improved water electrolyzer technology that does not require expensive metals such as platinum and iridium. And, there is a need to ensure that the water electrolyzers can turn off and on quickly in order to leverage the peaks and valleys associated with renewable energy sources, such as wind and solar, during peak periods of the day.

A Hydrogen filling station in Reykjavík, Iceland photo by Jóhann Heiðar Árnason. Licensed under CCBY2.0
Dioxide Materials’ high conductivity anion exchange membranes will be used to lower the cost of systems being used to create renewable fuels and chemicals and systems for energy storage. Illustrated in figure 1 is a run where a simple alkaline water electrolyzer with base metal catalysts (NiCo/Ni) is run at a constant current of 1 A/cm². Notice that we obtain 1 A/cm² at between 1.88 and 1.92 V. By comparison, the best previous alkaline water electrolyzers with base metal catalysts ran at currents below 0.25 A/cm² at 2.1V. Leng et al.7 were able to demonstrate alkaline cells running at 0.9 A/cm² at 2 V, but only when they used precious metals: i.e. iridium oxide anodes and platinum cathodes. These results demonstrate the potential of our membranes to substantially lower the cost of water electrolyzers. Presently, the water electrolyzers used in hydrogen filling stations and energy storage devices use precious metal catalysts (e.g. platinum/iridium). The results in Figure 1 show that one can replace the platinum and iridium with nickel and cobalt and get similar performance. Nickel and cobalt are much less expensive than platinum.
Dioxide's Technology Advantages include:
- Water electrolyzers that do not need precious metals (no platinum or iridium)
- Long lifetimes
- Reduced cost
- Rapid turn on/off
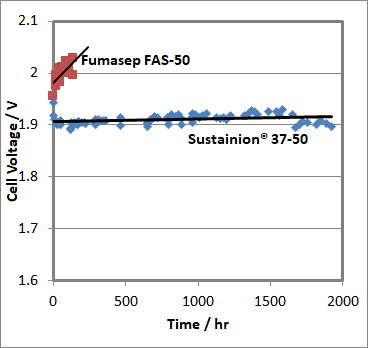
Figure 1 The voltage needed to maintain 1 A/cm2 in an alkaline anion exchange membrane electrolyzer operating at 60 °C in 1 M KOH.

