Dioxide Materials' Technology To Enable A Low Carbon Future
Lowering The Cost Of Renewable Energy
If one could replace electricity generated from fossil fuels with energy generated renewably, the world carbon footprint would be reduced. Unfortunately, solar and wind energy are intermittent. Solar panels produce the most energy when the sun shines brightly. Wind farms produce the most energy when the wind is blowing strongly. But, that might not be the time that the energy needs to be used. If the renewable sources produce more energy than can be used by the grid, the grid operator needs to limit (i.e. curtail) the amount of renewable energy that is accepted by the grid or else the grid will become unstable. So renewable energy suppliers have to shut down their solar or wind farm, or worse pay someone to take their energy. That raises the net cost of renewable energy.
When scaled, Dioxide Materials electrolyzers will be able to use hundreds of MW of electricity at one time. They can be turned on when renewable energy sources produce more energy than the grid can use for other purposes, and simply turned off when the energy can be used elsewhere on the grid. And, the electrolyzer system can make renewable gasoline and other products that will help lower the world's carbon footprint. Click Here for More Information

Renewable Fuels
Cars, trucks and airplanes are major sources of carbon dioxide emissions. If everyone switched to electric vehicles, and the electricity to charge the batteries came from renewable sources, the emissions would be eliminated. But such a change would take many decades.
Dioxide Materials' technology enables renewable gasoline to be made in the meantime.
The fuels we use today came from CO2 captured by plants. The plants use energy (sunlight) to convert CO2 and water into a reactive form: sugar or cellulose. Then, the reactive material is transformed at high pressures and temperatures underground to yield coal, natural gas, or petroleum.
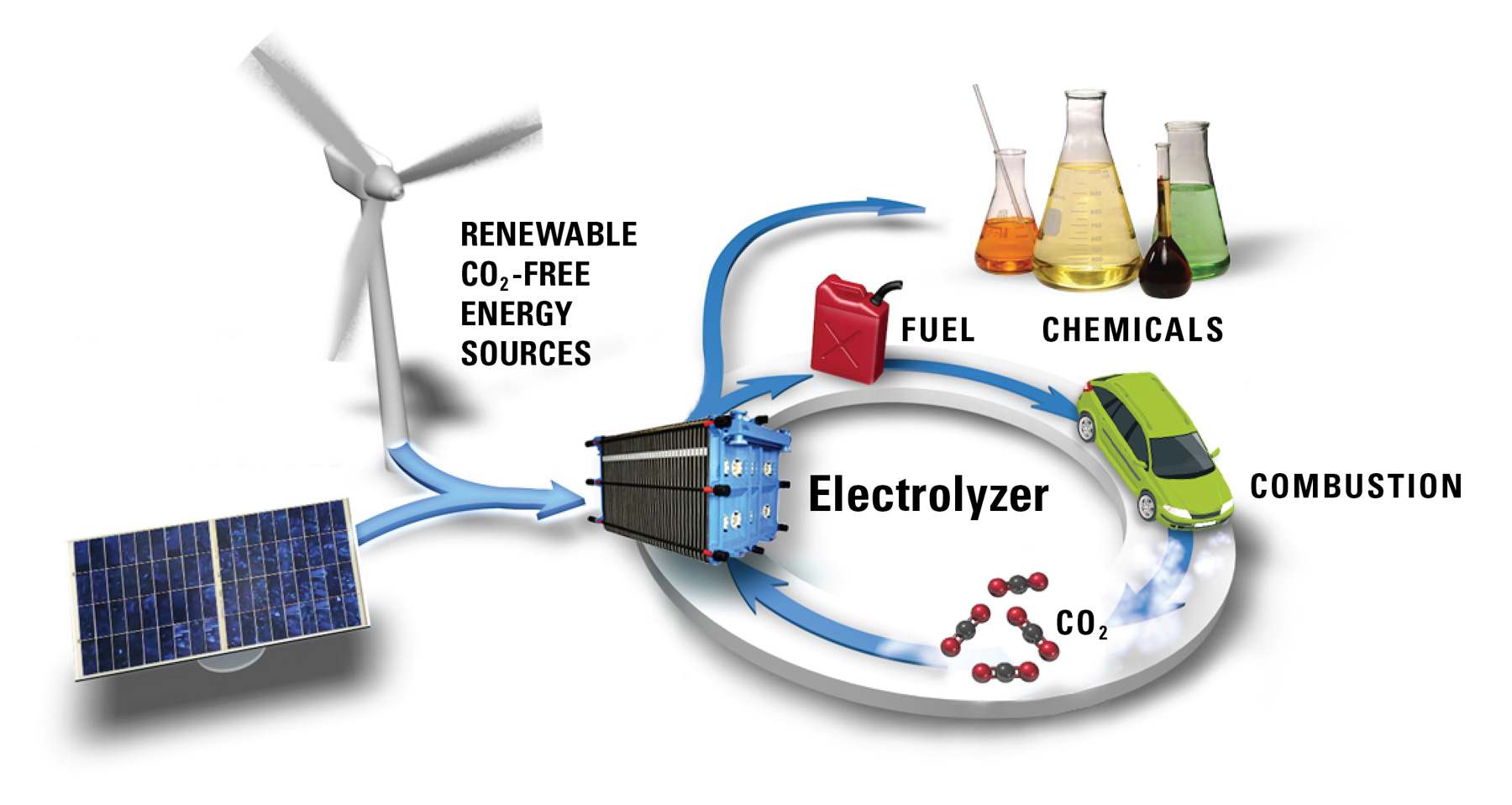
Dioxide Materials’ process is similar. First, renewable energy is used to convert CO2 and water into a reactive form called "syngas". Then, the syngas is transformed at high pressures and temperatures to create gasoline. The one big difference between Dioxide Materials’ process and the natural process is that we do everything faster. While it takes millions of years to produce oil, coal or natural gas underground, Dioxide Materials’ process does the same process in minutes. Also, Dioxide Materials’ process is much more efficient. While vegetation usually only converts about 1% of the captured energy into fuels, Dioxide Materials’ process is projected to convert about 60%.
Enabling the Hydrogen Economy
Hydrogen is the cleanest fuel available. Hydrogen can be made from natural gas, but electrolyzers are often used at hydrogen filling stations to make the hydrogen on-site.
Dioxide Materials is working to lower the cost of the electrolyzers. Most of the electrolyzers in hydrogen filling stations use expensive precious metals such as platinum or iridium, but Dioxide Materials is working on technology to replace the platinum and iridium with iron and nickel. We are also working to replace the expensive fluorinated membranes used now, with hydrocarbon based Sustainion® membranes. The result should be lower cost hydrogen filling stations, and a lower carbon economy. Click Here For More Information

Carbon Dioxide Recycling
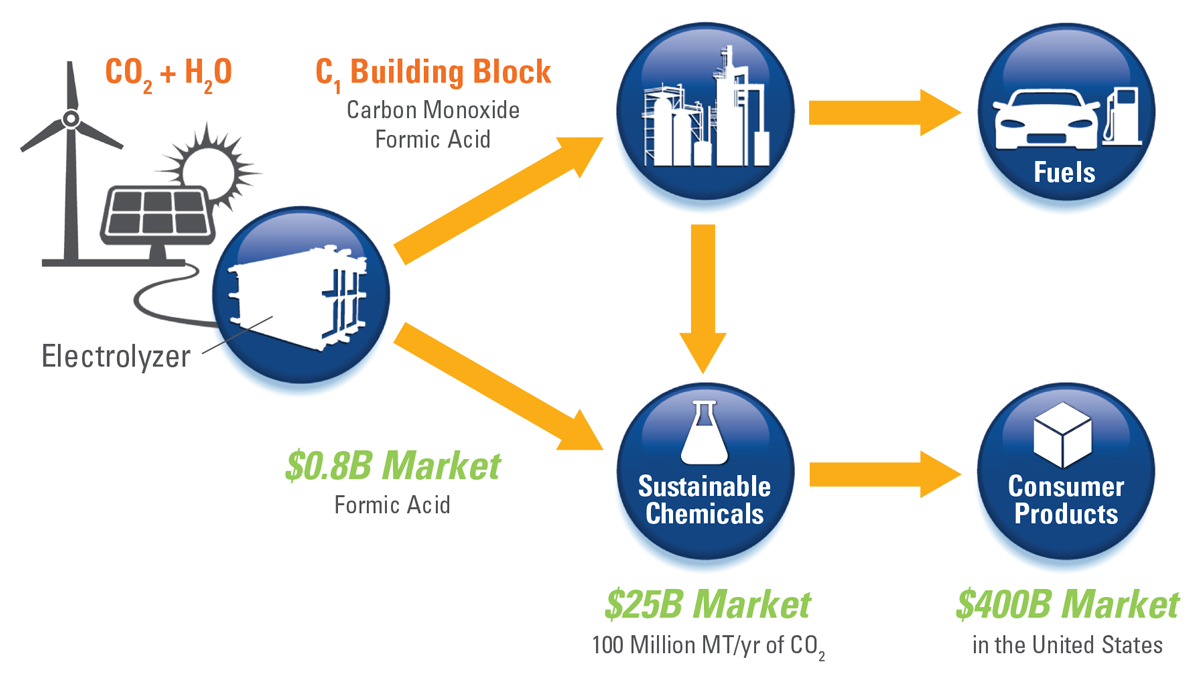
In the longer term, Dioxide Materials is working to make CO2 recycling a reality. The idea is to capture CO2 from the air and recycle it back to the fuels and chemicals that we use every day.
So far Dioxide Materials has concentrated on developing methods to convert the CO2 back to useful products, but CO2 capture will also be considered in our future work.