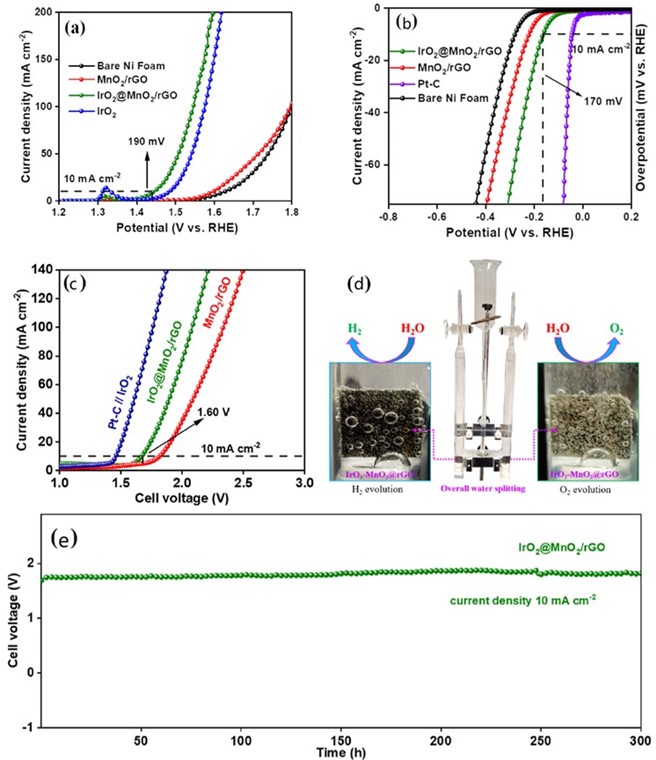
(a) OER polarization curves of IrO2@MnO2/rGO, MnO2/rGO, IrO2, and bare nickel foam (b) HER polarization curves of IrO2@MnO2/rGO, MnO2/rGO, Pt−C, and bare nickel foamin 1.0 M KOH at 1 mV s−1 (iR corrected) (c) Overall water-splitting device performance and (d) a picture of the device, (e) Chronopotentiometry curve for IrO2@MnO2/rGO||IrO2@MnO2/rGO at 10 mA cm−2 for 300 h.
Hydrogen is a promising future fuel with advantages of high specific energy density, and zero-carbon emissions. When coupled with renewable solar and wind energy, hydrogen production from water electrolysis holds the promise of low cost. Still, it is necessary to develop economical and efficient bifunctional electrocatalysts for water electrolysis. Recently, Yoo et al. (doi.org/10.1021/acssuschemeng.2c04074) developed a bifunctional electrocatalyst with ultra-low (~3.7%) loading of iridium oxide nanoparticles anchored on a hierarchical manganese oxide sheet grown in reduced graphene oxide (IrO2@MnO2/rGO). The optimized electrocatalyst shows enhanced bifunctional activity toward the oxygen evolution reaction (η10 = 190 mV) and the hydrogen evolution reaction (η10 = 170 mV) in a 1.0 M KOH electrolyte. The enhanced activity is attributed to a large surface area of hierarchical MnO2/rGO with a great number of IrO2 active sites and strong synergistic effect between IrO2 and MnO2. The fabricated IrO2@MnO2/rGO||IrO2@MnO2/rGO water-splitting device exhibits remarkably higher durability of about 300 h. The same device also displayed a lower cell voltage of 1.64 V at a current density of 10 mA cm−2. This provides an alternative pathway toward the design of an efficient, durable, and bifunctional electrocatalyst for clean hydrogen production and alkaline water/seawater electrolysis.
The above research used three-electrode half-cell and two-electrode H-cell and the reported potential and voltage were corrected by IR drop. Want to use zero-gap water electrolysis hardware without the need for the correction, please visit dioxide materials!