Anion exchange membrane (AEM) is considered as an alternative for proton exchange membrane (PEM) due to their potential utilizing platinum group metal (PGM) free catalysts in both fuel cells and electrolyzers. However, the true hydroxide conductivity of alkaline ionomers/membranes are not yet well-characterized partially due to their sensitivity to carbon dioxide (CO2) in air. Schwämmlein et al. (https://iopscience.iop.org/article/10.1149/1945-7111/ab8cdf/pdf) reported a method that reversibly converts hydroxide to (bi)-carbonate and vice versa (Figure 1) and evaluated the adaptability of current AEMs in severe environments, especially in freeze conditions.
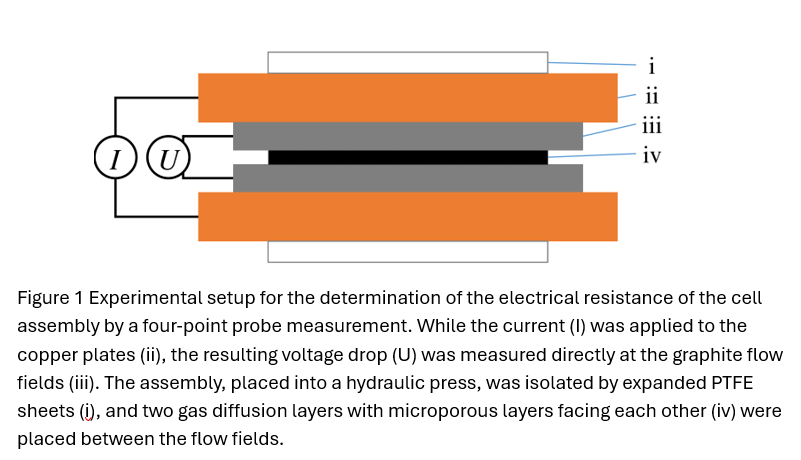
Both PEM and AEM were characterized in the form of catalyst coated membrane (CCM) prepared by decal transfer. A Tokuyama A201 anion exchange membrane based CCM was exchanged to bi-carbonate form, and sandwiched between two gas diffusion layers (GDLs), and installed in a cell hardware (Figure 1). The electrical resistance of the cell assembly was measured in the absence (Ra) and presence (Rp) of CCM using electrochemical impedance spectroscopy by a four-point probe configuration. The difference (Rp-Ra) is the membrane resistance (Rm) and used to calculate the conductivity. The membrane was converted from bicarbonate form to hydroxide form via electrochemical pretreatment with H2/O2, and back to bicarbonate form with air/air. Figure 2 shows the conductivity at different conditions.
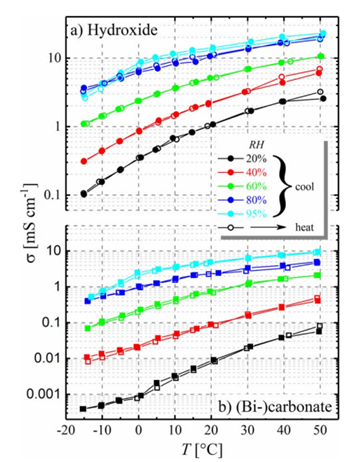
Figure 2 Through-plane conductivity, σ, of an MEA consisting of a Tokuyama A201 membrane equilibrated at 50°C with N2/N2 humidified at different RH values (as marked in the figure) as function of temperature, T, determined either in (a) the hydroxide form (circles) or in (b) the (bi-) carbonate form (squares). σ was calculated by subtraction of Ra. from the measured HFR and normalization to the thickness of the membrane (28 μm). The freeze cycle is shown as full symbols, while hollow symbols represent the thaw cycle. The hydroxide form was obtained by the carbonate purge approach, while the (bi-)carbonate form was obtained by a subsequent exposure to air/air.
The results show that the dynamic conductivity loss for AEMs at sub-freezing conditions is much larger than for PEMs. This poses potential challenges in the application of AEM fuel cells for electric vehicles but opens more research opportunities on AEMs. Moreover, AEM water electrolysis is promising to produce green hydrogen, helping decarbonization of our planet.
Want to know the true conductivity of your own AEMs, we have a conductivity cell made with low resistance graphite plates. Visit our website to learn more: https://dioxidematerials.com/product/cell-hardware-with-graphite-flowfields