Metal oxides experienced chemical transformation under operating conditions that limits their applications for photosynthesis. Liu et al. (doi.org/10.1038/s41560-021-00927-1) recently found that under illumination the Cu2O concurrently undergoes reduction by photoelectrons and oxidation by holes in the material. A protection scheme was proposed where an Ag catalyst was used to accelerate electron transfer and a Z-scheme heterojunction to extract holes. The resulting photoelectrode demonstrated stable CO2 reduction to ethylene with a 60% Faradaic efficiency, while bare Cu2O failed within minutes.
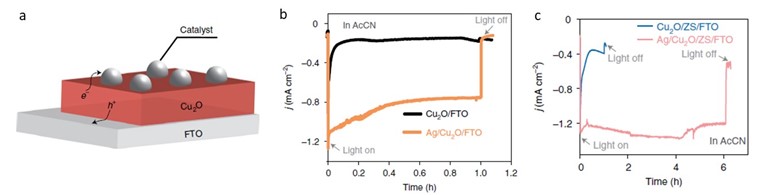
a) Stabilizing Cu2O with Ag catalyst and Z-scheme (ZS). b) Chronoamperometry of Cu2O and Ag/Cu2O photocathodes, c) Chronoamperometry of Cu2O/ZS/FTO and Ag/Cu2O/ZS/FTO photocathodes at −1.2 V versus Fc+/Fc. under AM 1.5G simulated sunlight (100 mW cm−2) using 0.1 M Bu4NPF6 as electrolyte and 0.1 M TEOA as proton donor in AcCN solution (CO2 saturated).
The experiments were conducted in aqueous (Na2SO4, KHCO3, NaOH and deionized water), and non-aqueous (acetonitrile) electrolytes. The results showed that the dissolved Cu ions in KHCO3 electrolyte are one order of magnitude higher than those in Na2SO4 in darkness. Under prolonged illumination, Cu0 and CuO/Cu(OH)2 were formed on the surface Cu2O in Na2SO4. In KHCO3 solution, significantly enhanced Cu2+-OH species are formed under illumination within 10 min. Cu2O showed rapid transformation into CuO/Cu(OH)2 within 10 min in NaOH, relative to Na2SO4 solution. In contrast, Cu2O barely changed without any solutions present or in acetonitrile non-aqueous solution under illumination for 4 h. This validate the hypothesis that the presence of OH− ions in aqueous solutions may be key in promoting the oxidative transformation of Cu2O under illumination. The results show Cu2O degradation depends on the electrolyte and applied potentials.
Want to see how Cu2O performs in solid electrolyte (no liquid electrolyte), try Dioxide materials’ Sustainion anion exchange membrane electrolyte.