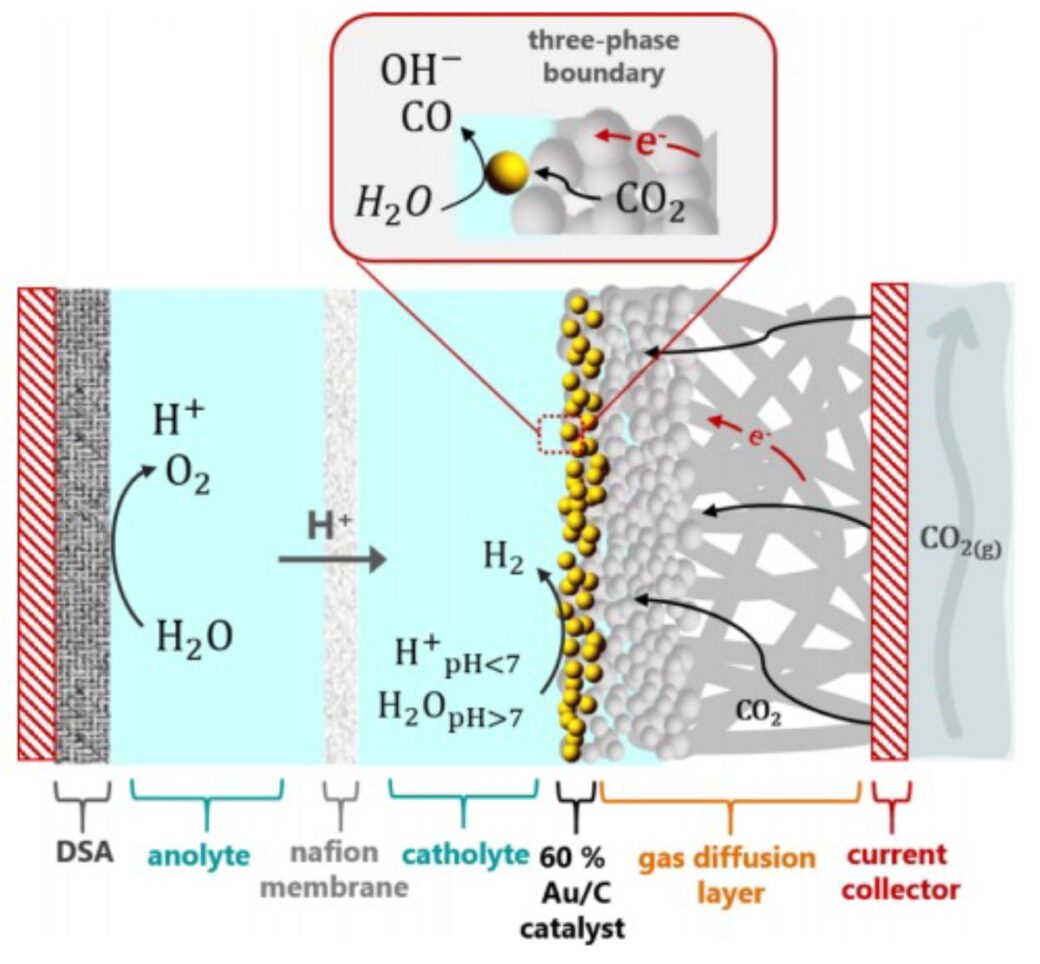
Fig. 1 CO2 electrolysis. Schematic representation of the gold gas diffusion electrode system
CO2 electrolysis has mainly been performed in neutral or alkaline media, but some fundamental work shows that high selectivities for CO can also be achieved in acidic media. A recent paper, published in nature communications (doi.org/10.1038/s41467-021-24936-6), reported that CO2 electrolysis can operate at pH 2-4 in sulfate electrolyte, and achieved current densities up to 200mA/cm2 and CO faradaic efficiencies between 80-90%, with a 30% improvement of the overall process energy efficiency in comparison with neutral media. Additionally, the weakly hydrated cations are crucial for accomplishing high reaction rates and enabling CO2 electrolysis in acidic media. This study represents a step towards the application of acidic electrolyzers for CO2 electroreduction.
It was generally accepted that proton reduction can be fully suppressed in acidic media, as long as the rate of CO/OH− formation from CO2 reduction is HIGH enough to compensate the mass transfer of protons to the electrode surface. To make this happen, high surface area electrodes rather than flat electrodes were used to generate high current. Figure 1 shows the schematic of the gas diffusion electrode system. The results show that there is clear competition between CO/OH– formation and proton diffusion from bulk electrolyte to the electrode/electrolyte interface. 90% CO faradaic efficiency was achieved at 100mA/cm2 and pH 3 and 4 (Figure 2).
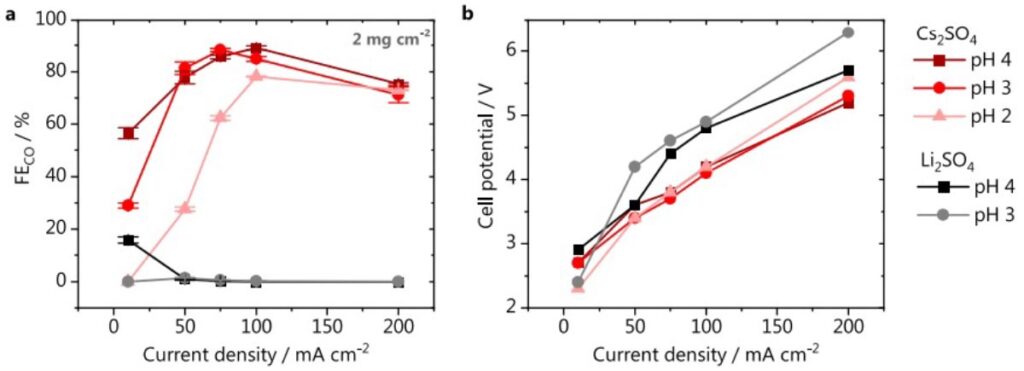
Fig. 2 Effect of pH and cation identity on CO2 electrolyis. a Faradaic efficiency for CO and (b) cell potential. Electrolysis performed in either 1 M Cs2SO4 (red) or 1 M Li2SO4 (gray scale), catalyst loading 2 mg cm−2.
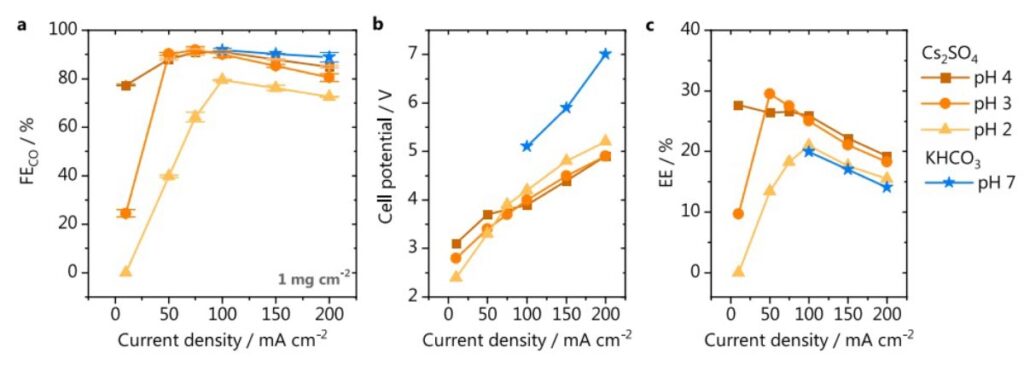
Fig. 3 Comparison between acidic and neutral media. a Faradaic efficiency for CO; (b) cell potential and (c) energy efficiency for CO2 electrolysis performed in either 1 M Cs2SO4 (orange) or 1 M KHCO3 (blue stars), catalyst loading 1 mg cm−2.
Reducing the catalyst loading improved CO faradaic efficiency slightly at higher current and pH 2-4. It seems that CO faradaic efficiency is less dependent on the current densities at pH 7 in 1M KHCO3. However, the cell voltage is much higher at pH 7 in 1M KHCO3 than at pH 2-4 in 1M Cs2SO4,leading to 30% lower energy efficiency. This is at least partially due to the lower conductivity of 1M KHCO3.
In summary, this study shows that reduction of CO2 to CO in acidic media can considerably lower the cell voltage, and consequently improve the energy efficiency as compared to that in neutral media. Under the industrially relevant conditions, the high rate of CO2 reduction (i.e., high current density) leads to the production of sufficient OH− to neutralize the protons before they reach the catalyst layer. This hinders proton reduction and enables a high selectivity for CO even at bulk pH 2, at 200 mA cm−2. This work lays down a new path toward the development of acidic CO2 electrolyzers. [The views and opinions expressed in this article are those of the authors and do not necessarily reflect the DM’s.]
However, it is worth to note that high CO faradaic efficiency is only achieved at specific current densities and pH. At lower current densities, the rate of CO/OH– formation is too low to compensate the proton diffusion from bulk electrolyte to the electrode/electrolyte interface, so the CO faradaic efficiency is low. At high current densities, CO2 supply to the electrode surface is limited due to the CO2 diffusion limitation associated with liquid electrolyte, so the faradaic efficiency is also low. Furthermore, the cell voltage is >4 and >5V at 100 and 200mA/cm2, corresponding to energy efficiency of only 25 and 20%, respectively. These need to be tackled for CO2 electroreduction in acidic media. [The views and opinions expressed in this paragraph are solely DM’s and do not necessarily reflect the authors of the article.]