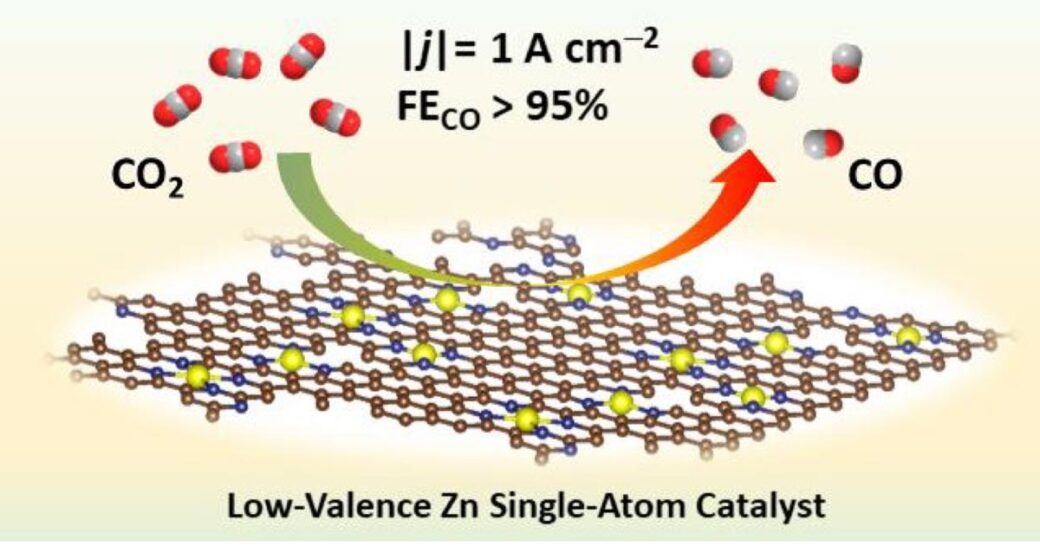
The electrochemical CO2 reduction reaction (eCO2RR) represents a promising solution for mitigation of excessive CO2 concentration in the atmosphere as well as for utilization and transformation of CO2 into value-added chemicals and commodities. This technology, if powered by renewable energy such as solar and wind power, has great potential to reach industrial level and achieve long-term energy storage. However, the inert nature of the CO2 molecule and the kinetically more favorable competing hydrogen evolution reaction (HER) lead to low energy utilization and poor selectivity toward the target products. Thus, it is important to improve the scalability of eCO2RR by designing affordable catalysts that overcome the challenges regarding activity and selectivity. In a recent work published in Angew. Chem. Int. Ed. (“Low-Valence Znδ+(0<δ<2) Single-Atom Material as Highly Efficient Electrocatalyst for CO2 Reduction” https://doi.org/10.1002/anie.202107550), Daasbjerg and coworkers reported a low-cost nitrogen-stabilized low-valence Zn-based single-atom catalysts (SACs) that shows record-high current density of 1A cm-2 with CO selectivity of 95%. The authors believed that the developed Znδ+-NC catalyst shows great prospect for industrial applications.
In this work, Znδ+-NC catalysts were prepared through multiple steps including coordination and carbonization processes. The Zn content was determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES). The coarse structure of the catalyst and the crystallinity were checked by scanning electron microscopy (SEM) and powder X-ray diffraction (XRD) spectroscopy, respectively. Additionally, transmission electron microscopy (TEM) and high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images revealed that Znδ+-NC possesses thin nanosheets morphology with uniform distribution of C, N, and Zn and the existence of Zn single atoms in Znδ+-NC. The authors then employed other technologies including X-ray photoelectron spectroscopy (XPS), X-ray
absorption near-edge spectra (XANES) and extended X-ray absorption fine structure (EXAFS) to characterize chemical composition and elemental state of Znδ+-NC. The observations suggest the existence of non-centrosymmetric and unsaturated Zn-N centers in Znδ+-NC. Specifically, the results reveal that Znδ+-NC consists of a mixture of Zn(II)-N4 and Zn(I)-N3 sites, leading to a mix-coordinated and low-average-valence Zn-Nx (3≤x≤4) structure while the Zn-N3 and/or Zn-N3-V could hold enough delocalized electrons to facilitate electron transfer and affect the intermediate adsorption/desorption steps during eCO2RR.
Next, eCO2RR activity and selectivity of the catalyst were evaluated in an H-cell at selected potentials vs the reversible hydrogen electrode (RHE). It was found that Znδ+-NC gives a CO faradaic efficiency (FECO) as high as 99% at a fairly low overpotential of 310 mV. The stability of Znδ+-NC was then examined by carrying out continuous CO2 reduction at -0.57 V vs RHE for 12 hours. It was found that both FECO (95.3%) and current density (0.9 mA cm-2) remained stable over the entire period and negligible changes to Znδ+-NC were observed. So the authors claimed that the results validated the superior durability and stability of single-atom Zn sites for eCO2RR. Further, the electrocatalytic activity of Znδ+-NC towards eCO2RR was attributed to the unsaturated Zn-N3 and Zn-N3-V sites.
Last, the potential of the Znδ+-NC catalyst for industrial applications were assessed by using a flow cell with Sustainion® X37-50 anion exchange membrane and applying 1 M KOH as electrolyte. The testing results showed that FECO remained at 95 ± 4% while increasing current density from 0.2 to 1 A cm-2. With the obtained results, the authors believed that the work not only shed light on the relationship among coordination numbers, valence state, and catalytic performance of Zn single atom sites, but also serves as guidelines for designing and developing such catalysts to achieve high current densities relevant for industrial applications. This work provides a promising strategy of developing low-cost, highly efficient, and stable catalyst for eCO2RR. However, the catalyst was only tested for 12 hours for long-term stability at constant potential (-0.57 V vs RHE), similarly, testing in the flow cell at different current densities only last for 600 s and 1 h, which are not sufficient at all for long-term stability evaluation in both cases. Additionally, although the highest current density obtained was 1 A cm-2, however, it was achieved with cell voltage higher than 7 V in the presence of 1 M KOH electrolyte! This may not be economically plausible for industrial applications. Therefore, works including more detailed studies, catalyst optimization, and comparison with benchmark catalyst performance need to be done before drawing any conclusions of the potential industrial applications of the proposed catalyst.