There is a growing interest in the conversion and storage of renewable solar energy via reduction of carbon dioxide into clean fuels, as an alternative to fossil fuels. Photoelectrochemical (PEC) devices, which use semiconducting photoelectrodes to absorb solar energy and drive uphill chemical reactions, constitute a promising approach to achieve this goal. However, both photoanodes and photocathodes usually suffer from catastrophic degradation under operating conditions. Such degradation has prevented the development of photoelectrochemical systems as a viable alternative for energy conversion and storage. It is crucial to understand the chemical transformation in the photoelectrodes under illumination to enhance the electrode stability. In a recent paper published in Nat. Energy (Nature Energy, 2021, 6, 1124-1132, https://doi.org/10.1038/s41560-021-00927-1), Liu and co-workers used a correlative approach to unravel how cuprous oxide (Cu2O) photoelectrodes change under reaction conditions and provide a protection scheme to mitigate degradation.
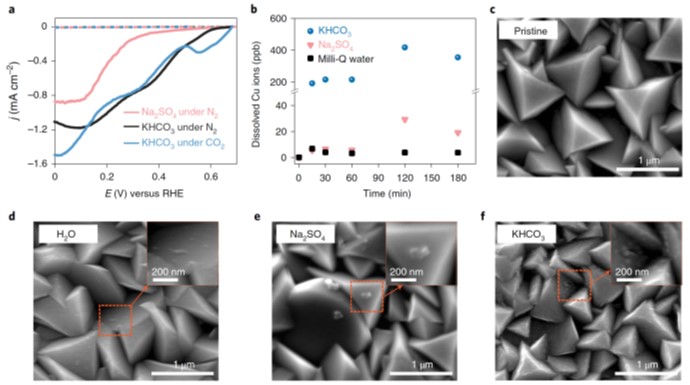
The authors first studied electrolyte dependent Cu2O degradation in 0.1 M Na2SO4 and KHCO3 electrolytes in the dark. It was found that Cu2O dissolves in aqueous solutions. The photocurrent of Cu2O with CO2 observed in different electrolytes stems from varying corrosion rates of Cu2O with specific supporting electrolytes, instead of the Faradaic current towards HER or CO2RR. Based on the characterization results, the authors believed that while Cu2O suffers from chemical degradation when contacted with aqueous solutions in the dark, the specific interaction of Cu2O with each surrounding electrolyte may be the key in defining the kinetic pathway of Cu2O transformation under illumination. The authors then conducted studies on the transformation mechanism of Cu2O under illumination. The results indicate pronounced Cu2O oxidation under illumination. Additionally, Cu2O reduction under cathodic biasing operation was also observed. The Cu species during oxidation (Cu2+-OH) and reduction (Cu0) were then identified. The authors decided that inhibiting the formation of Cu2+-OH species in aqueous solutions is of primary importance. To address such issue, the authors designed a Z-scheme (ZS) and combined a Ag catalyst. The concurrent integration of the Ag catalyst, the ZS heterostructure with Cu2O allows the developed catalyst achieved stable photocurrent. The integrated catalyst also showed good catalytic activity toward CO2RR. The PEC system provides good selectivity and sustainable production of ethylene (C2H4, FE ~60%) over time.
This work suggests that future development of strategies to incorporate Cu2O in new architectures and environments may open new avenues for rational design of Cu2O in solar-driven devices. The correlative approach and protection strategies described here could also be extended to other photoelectrocatalytic materials.