Membrane-separated electrochemical reactors have the potential to improve chemical manufacturing practices as they can lead to significant enhancements in energy conversion efficiency and reductions in manufacturing costs. The ion exchange membranes used in such reactors can (i) help prevent reactions of products and reactants in opposite chambers, (ii) allow the independent optimization of cathodic/anodic reaction conditions, and (iii) reduce the interelectrode distance in an overall effort to lower energy losses, (iv) reduce the downstream separation costs. Therefore, membrane-separated electrochemical reactors will play a significant role in the transition from heat-powered to electricity-driven chemical processes, helping to accelerate the electrification of the chemical industry.
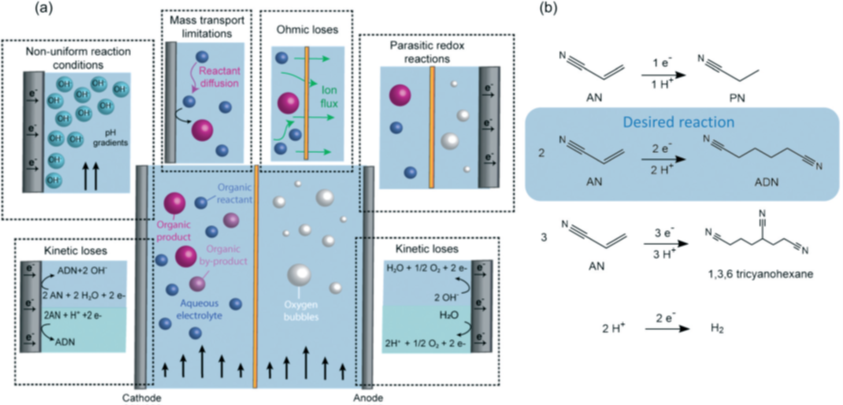
In electrochemistry, while the use of membranes has been extensively investigated in inorganic processes, their use in organic electrosynthetic applications has remained relatively unexplored. Motivated by the possible enhancement in energy conversion efficiencies with the incorporation of membranes in organic electrosynthetic reactors, Modestino and coworkers systematically explored the effect of organic-containing electrolytes on some of the most common commercially available CEM (Nafion) and AEMs (Sustainion and Fumasep FAB), using the electrosynthesis of adiponitrile (ADN) as a model reaction. This work was published in React. Chem. Eng. (React. Chem. Eng., 5, 136, 2020, DOI: 10.1039/c9re00389d).
The electrosynthesis was conducted using two different reactors: undivided flow reactors, and reactors divided by CEM or AEMs. The highest selectivity toward ADN reached with the undivided flow reactor was 48% at 40 mA cm-2 and 4.0 V cell voltage, which is significantly lower than the 83% reached in a membrane separated batch reactor. The performance limitations could arise from the slow charge transport in the electrolyte, large kinetic losses, non-uniform conditions throughout the electrode surface, and possible oxidation of organic species at the anode. For Nafion-separated flow reactor, the experiment results showed decrease in crossover, increase in ion-conductivity and reaction kinetics, which result in a significant improvement in ADN selectivity (77% at 20 mA cm-2 and 2.6 V cell voltage) compared to undivided cells. Both AEM-separated flow reactors saw improved kinetics and decreased organic species crossover, which helped to improve ADN selectivity with respect to undivided reactors. The highest selectivity of 81% was obtained with Sustainion membrane at 40 mA cm-2 and 3.0 V applied voltage.
This study revealed that membrane permeability, conductivity, and electrolyte pH are significant drivers for energy efficiency and selectivity in membrane-separated reactors, and membrane properties can be severely affected by the presence of organic reactants in the electrolyte. The insights provided in this study are an important step towards developing design guidelines for continuous organic electrolsynthetic processes which will contribute to the deployment of scalable and sustainable manufacturing in the chemical industry.