Electrochemical CO2 reduction reaction (CO2RR) provides a promising route to convert renewable electricity into valuable and storable multi-carbon products such as ethanol. With recent advances in CO2RR catalysts, the electroproduction of ethanol approaches industrially relevant production rates and selectivities. Membrane electrode assembly (MEA) electrolyzers are reaction platforms that, when equipped with highly active and selective catalysts, enable CO2-to-ethanol conversion at high current densities and selectivities. In practice, ethanol-producing MEA systems have suffered liquid product crossover. Concentrated liquid products formed at the cathode migrate through the membrane—via electroosmotic drag and diffusion—to the anode side where they are either oxidized or diluted in the bulk anolyte. At industrially relevant reaction rates (>100 mA/cm2), up to 75% of the ethanol produced at the cathode passes through the AEM limiting the recoverable ethanol yield. The ethanol concentrations in the anolyte are generally in the range of 0.05 wt %. Recovering ethanol from such low concentration streams is not practical, incurring a downstream separation energy penalty that exceeds the product value. On the other hand, industrial bioethanol processes provide ethanol at 10 wt %, setting a benchmark concentration for electrocatalytic production that is 200-fold that of current electrolyzer anolytes. Therefore, increasing product stream concentrations and reducing energetic losses are critical for the feasibility of CO2-to-ethanol conversion. Aimed at addressing this challenge, Miao and coworkers from University of Toronto reported an electrolyzer system that simultaneously blocks ethanol crossover and enables its direct collection from the cathode at concentrations exceeding 10 wt %. Their work was recently published in Joule (“Electrooosmotic flow steers neutral products and enables concentrated ethanol electroproduction from CO2” https://doi.org/10.1016/j.joule.2021.08.013).
To establish a baseline and assess the ethanol crossover, the authors first performed experiments in the conventional MEA electrolyzer with an AEM (anion exchange membrane). The degree of crossover was measured in a current density range from 100 to 300 mA/cm2 with 0.1 M KHCO3 anolyte. The selectivity toward ethanol, the CO2RR products at the cathodic and anodic streams, the total ethanol faradic efficiency (FE) at different current densities were then measured and calculated. It was found that the total ethanol FE increased with increasing current density and reached a maximum FE of 23% at 250 mA/cm2. The degree of ethanol crossover remained relatively constant and exceeded 75% at all current densities studied. This result confirmed that most of the produced ethanol migrated through the AEM to the anode and was diluted in a large volume of anolyte. The concentration of ethanol in 200 mL anolyte was determined to be less than 0.05 wt % after 5 h of continuous electrolysis at 200 mA/cm2. Ethanol produced at this concentration is not practical to recover and is a waste steam. The concentration of ethanol in the anolyte could be increased by extending the electrolysis duration or decreasing the total volume of anolyte. However, based on the control experiments of ethanol oxidation on anode at different concentrations, the ethanol concentration in the anolyte will be limited to less than 0.5 wt % due to ethanol oxidation, which is a concentration well below the practical threshold of 10 wt %.
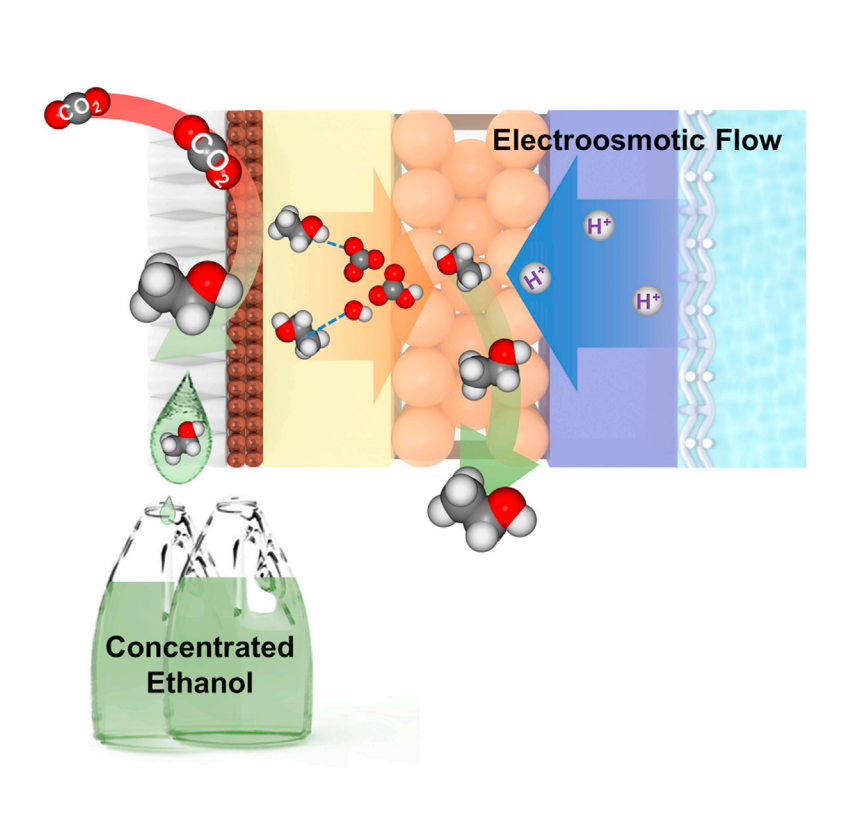
To mitigate the crossover of ethanol from the convection and diffusion, the authors employed a porous proton exchange layer placed between the AEM and a cation exchange membrane (CEM) next to the anode. Such configuration allowed direct ion conduction from the anode to cathode in the direction opposite to ethanol crossover and facilitated the collection of ethanol that migrated across the AEM. At the same time, the CEM ensured the proton flux and prevented direct mixing of the liquid products with the anolyte.
The experiments with such cell configuration were then carried out to evaluate the performance. First, it was found that the porous proton exchange layer did not alter the cathodic reaction environment significantly, similar gas and liquid products were obtained. Second, ethanol crossover to the anode side was largely blocked, with <1% of produced ethanol detected in the anolyte over the full range of current densities from 100 to 300 mA/cm2. The authors then found that the ethanol concentration in the porous layer can be controlled by supplying either DI water or inert N2 gas through the layer. Decreasing the flow rate of DI water or tuning the flow rate of N2 in the porous layer allowed the production of a higher concentration of ethanol in the porous layer. The performance could be further improved by adjusting the operating temperature. With the operating temperature of 40 °C, a cathodic ethanol concentration of 10.5 wt % was achieved. The electrolyzer stability was then evaluated at 200 mA/cm2 current density, with a DI water flow rate of 0.05 mL/min through the porous layer, 0.01 M H2SO4 as the anolyte, and the Sustainion® X37-50 membrane. The electrolyzer could produce ethanol with a stable concentration of 7.5 wt % for over 80 h of continuous operation. By using a thinner proton exchange layer, substituting the Sustainion® X37-50 membrane with a low-water-uptake AEM, a maximum ethanol concentration of 13.1 wt% from the cathode was achieved. However, the performance was only maintained for 20 hours.
In this work, the authors reported an approach that eliminates the ethanol crossover loss that has limited the electrochemical CO2 conversion to ethanol. Applying a proton exchange layer in the electrolyzer blocks the electroosmotic and diffusive transport and eliminates the crossover of ethanol to the anode, resulting in less than 1% ethanol loss to the anode and exceeding 10 wt % ethanol at the cathode. The strategy reported in the work provides a viable route to extract liquid product at useful concentrations from CO2 electrolysis. However, further improvements on electrolyzer voltage and, especially, long-term stability are needed for future studies.