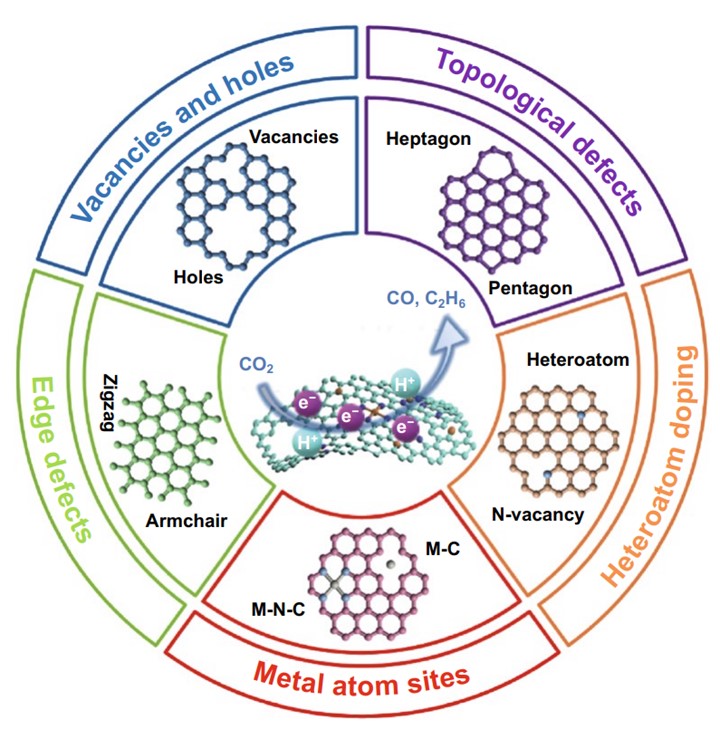
Electrocatalytic carbon dioxide (CO2) reduction (ECR) holds the potential to close the carbon cycle and store renewable energy. However, there are still some problems such as low activity and selectivity, and poor stability. One of the most promising strategies to improve ECR activity is to develop electrocatalysts with low cost, high activity, and long-term stability. Recently, defective carbon-based nanomaterials have attracted extensive attention due to the unbalanced electron distribution and electronic structural distortion caused by the defects on the carbon materials. This review article (https://doi.org/10.1007/s40820-020-00538-7) summarizes the latest research progress of the construction of the diverse types of defects (intrinsic carbon defects, heteroatom doping defects, metal atomic sites, and edges detects) for carbon materials in ECR, and unveil the structure–activity relationship and its catalytic mechanism.
Table 1 compares the performance of different carbon-based catalysts for ECR. Noted that most experiments were conducted in aqueous electrolyte either in H-cell or in flow-cell, and durability tests were less than 100hr. Dioxide Materials’ patented unique anion exchange membrane CO2 electrolyzer has eliminated liquid electrolyte in gas-phase CO2 reduction and demonstrated over 3000hr stable performance. Want to achieve over 1000hr stable performance with your own catalysts? Try Dioxide Materials’ Sustainion® membrane and cell hardware!