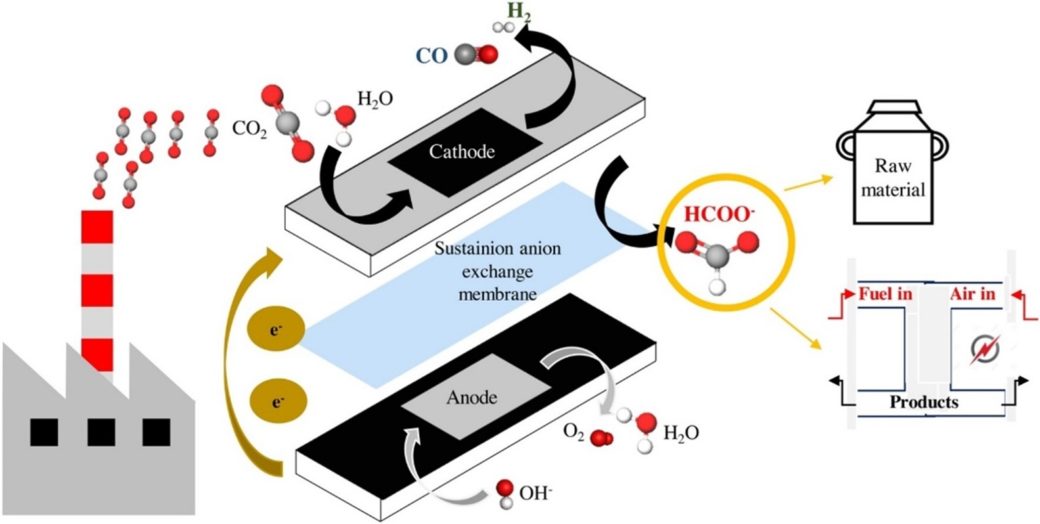
The electrochemical conversion of CO2 to formic acid or formate has attracted great interests recently. There is a large number of studies published in this field. However, most of the studies operated in a discontinuous mode in different electrochemical reactor configurations which is not feasible for industrial applications. Further, many studies were conducted with a current density lower than 100 mA cm-2 or 200 mA cm-2, which is required for future implementation of the electrochemical process at the industrial scale. In this context, Diaz-Sainz and co-workers recently published a paper compiling recent research advancement in this field and their studies on the CO2 conversion to formate in a continuous mode with a current density up to 600 mA cm-2 (https://doi.org/10.1016/j.jcou.2021.101822).
The authors first summarized results reported in literature for CO2 conversion to formic acid or formate in a continuous mode of operation at current densities higher than 200 mA cm-2. Based on the literature works, the authors observed that most of studies use liquid electrolyte at the cathode side of the electrochemical reactor, which leads to limited electrochemical performance due to the limited CO2 solubility. In addition, the type of ionic exchange membrane and electrochemical reactor configuration also play important roles in the performance of the electrochemical conversion of CO2 to formic acid and formate. Therefore, the authors employed a continuous filter press reactor with a single pass of the reactants through the reactor in this study. They used Dioxide Materials’ Sustainion® anion exchange membranes (AEMs) in the reactor for all the testing. For cathode selection, Bi-based cathodes were used in this study. To avoid liquid electrolyte, humidified CO2 gas stream was fed into the cathode side.
The testing results indicated that excellent values of formate Faradaic efficiency (>90%) were obtained at the current density from 90 to 300 mA cm-2, while it decreased fast to around 75% with 600 mA cm-2 current density. The energy consumptions of less than 200 kWh kmol-1 were also achieved at current densities of 90 to 200 mA cm-2. The authors also compared the testing results using Sustainion® anion exchange membranes and Nafion membranes, which were usually used in most of the studies in literature. It was observed that higher current densities were obtained with Sustainion membranes. And the energy consumption was clearly improved by 15% when Sustainion membranes were used with respect to Nafion.
The novel filter press electrochemical reactor using Sustainion anion exchange membranes shows similar performance to the GDE configuration with liquid electrolyte but with the advantage of using humidified CO2 as an input to the cathode side. The obtained results show excellent combinations of Faradaic efficiency for formate, energy consumption, and product rates. The configuration with Sustainion membranes can be particularly interesting for future applications that do not involve a very concentrated formate product. Still, studies on cathode catalyst loading effect, improvement of performance at higher current densities, and long-term performance evaluation will be needed for future work.