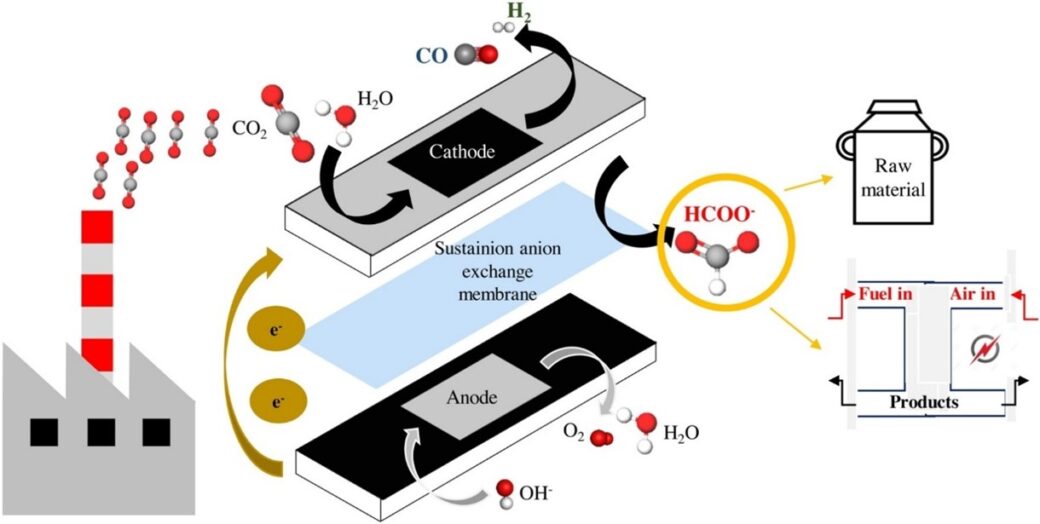
Electrochemical CO2 reduction to formic acid or formate is considered as a promising approach to convert CO2 to value-added product and a strategy of mitigating climate change. Recently, Diaz-Sainz and coworkers developed a novel electrolyzer cell configuration which allows the electrolyzer to run at a high current density with promising Faradaic Efficiency, energy consumptions, and product rates. Their work was published in Journal of CO2 Utilization (https://doi.org/10.1016/j.jcou.2021.101822).
There is a large number of published studies in CO2 electroreduction to formic acid/formate operating in a discontinuous mode in different electrochemical reactor configurations, and under a current density lower than the one required for the industrial scale (i.e. at least 100 mA cm-2, even 200 mA cm-2). To bring the CO2 electrochemical process closer to industrial applications, the use of a continuous electrolyzer operating at high current density is the desired operation mode. In this work, continuous operation was employed which enhances the CO2 electroreduction results in terms of Faradaic Efficiency and energy consumption. To avoid issues caused by using an aqueous catholyte such as low solubility of CO2, cost of the ionic compounds, the authors also employed a CO2 humidified input stream at the cathode side instead of using an aqueous electrolyte.
The authors then designed a filter press cell configuration which includes a Bi-C/GDE cathode, an IrO2 MMO anode, a Sustainion® RT 37-50 anion exchange membrane to separate cathode and anode chambers. The anode side was fed with 1 M KOH solution while the cathode size was fed with humidified CO2 gas.
The testing results showed that the cell can be operated at current densities up to 600 mA cm-2 with an excellent combination of formate Faradaic Efficiency and energy consumption. For instance, the Faradaic Efficiency was 91.6% at 300 mA cm-2 current density, decreasing by only 4% compared to that at 90 mA cm-2 current density. For energy consumption, it was only 196 kWh kmol-1 at 200 mA cm-2 current density, largely due to the low cell voltage achieved. However, when operating at higher current densities such as 500 and 600 mA cm-2, the formate Faradaic efficiency and energy consumption started to worsen, as well as the formate production rate.
The authors attributed the competitive values in Faradaic Efficiency, formate production rate, and the energy consumption to the fact that Sustainion® anion exchange membranes exhibit an excellent performance and has a very low area-specific resistance under alkaline conditions. Therefore, the cell configuration with the Sustainion® membrane studied through this work can be particularly interesting for future applications that do not involve a very concentrated formate product. In addition, these results present a significant advance in the field of CO2 electrochemical reduction to formate with the potential applications for future implementation at the industrial scale.