Electrochemical CO2 reduction is a promising strategy to store renewable electrical energy in the chemical bonds of carbon fuels. Currently, high performance has been reported for C1 products, like CO and formate. However, C2 products such as ethanol are produced competitively with ethylene, due to shared reaction pathways. Enhanced ethylene production has been achieved by implementing Cu-based catalysts via materials engineering. However, efficient ethanol production by CO2 reduction remains a major challenge. In a recent paper published in PNAS (PNAS 2023, 120 (4), e2214175120, https://doi.org/10.1073/pnas.2214175120), the authors developed a Cu/Au bimetallic catalyst to improve the ethanol selectivity over ethylene. The results show that both improved ethanol selectivity and current density were achieved.
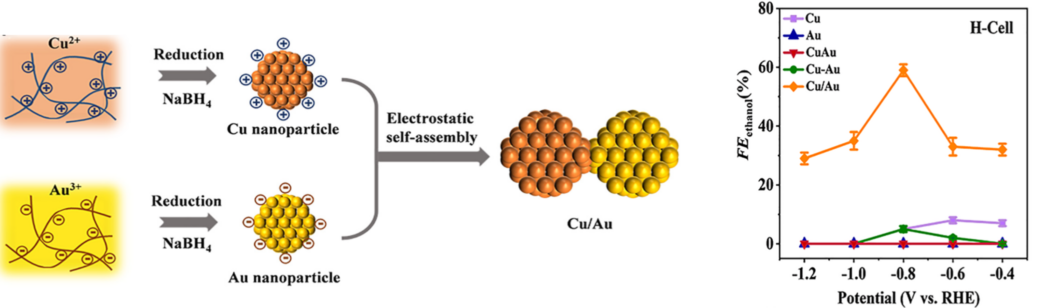
The authors first utilized the density functional theory (DFT) to examine the possible reaction pathways on Cu/Au heterojuctions based on previous studies on Cu-based catalysts. The DFT calculation indicates that Cu/Au heterojuctions appears to give C2H5OH by asymmetric hydrogenation of OCCO* through the intermediate OCCOH*. This finding suggests that Cu/Au heterojuctions play a major role in ethanol generation during the reduction of CO2. Based on the DFT results, the authors then fabricated Cu/Au heterojuctions through well-designed electrostatic self-assembly of positively charged copper and negatively charged gold nanoparticles. Structure characterizations confirmed the formation of Cu/Au heterojuctions.
The CO2 electrochemical reduction on Cu/Au heterojuctions was then evaluated in both H-cell and flow cell. The obtained results in a H-cell showed that ethanol was the main C2 product on Cu/Au heterojuctions with a maximum ethanol Faradaic efficiency reached at -0.8 V, while only trace amount of ethanol was detected on Cu and separated Au-Cu catalysts. The Faradaic efficiency ratio of ethanol over ethylene on Cu/Au heterojuctions was significantly enhanced by a factor of 200 compared to Cu at -0.8 V (83 vs. 0.41). When tested in flow cell, the ethanol partial current density of -300 mA cm-2 on Cu/Au heterojuctions, at -0.75 V vs. RHE, is a factor of 4 higher than that at Cu. An average current density of -500 mA cm-2 at -0.75 V was obtained during a 90 h long term test. Evidently, the Cu/Au heterojuctions output the highest ethanol partial current density among the reported catalysts. In addition, the cathode energy efficiency was calculated to be 39.8% at an applied potential of -1 V vs. RHE without considering any IR compensation.
The study described an optimized procedure for the electrocatalytic reduction of CO2 to ethanol based on the rational design and modification of bimetallic catalysts. The electrocatalytic results show that Cu/Au heterojuctions can lead to the selective conversion of CO2 to ethanol with improved Faradaic efficiency and current density. The overall results highlight that the potential utilization of Cu/Au heterojuctions for the formation of ethanol can occur under conditions appropriate for industrial production. However, more studies such as the long-term stability test of the catalyst and tests in CO2 electrolyzers are needed to further evaluate the developed Cu/Au heterojuctions catalyst.