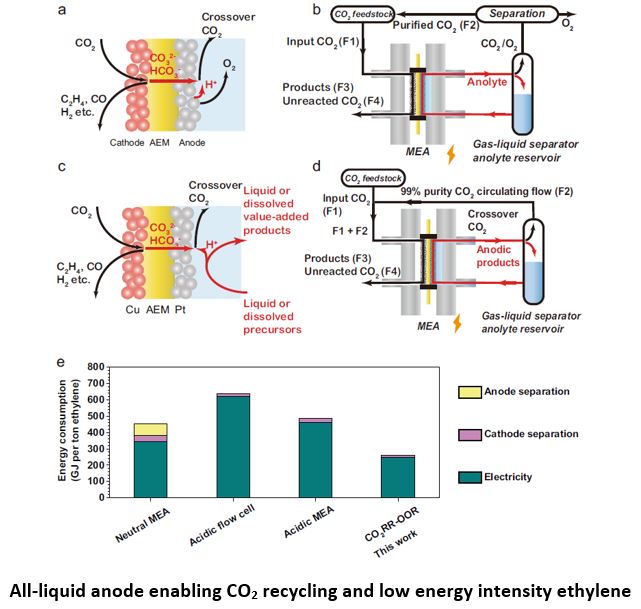
At present, electrochemical reduction of CO2 is favored at cathode under highly alkaline conditions while O2 is produced via oxygen evolution reaction (OER) at anode. Unfortunately, carbonate is formed under these conditions, consuming both OH– and CO2 thus lowering the local pH and carbon efficiency. Anion exchange membrane, separating cathode and anode of the electrolyzer, enables a high local pH at cathode and mitigates CO2 loss simultaneously. Still, a significant amount of CO2 reacts with OH– to form carbonate at cathode which crosses the anion exchange membrane to the anode. As a result, CO2 is released together with O2 produced at anode, producing a gas mixture of CO2 and O2. The separation of CO2 from O2 is needed to improve the carbon efficiency, but the energy penalty is prohibitive at this moment. Recently, Sargent et al report a liquid-to-liquid anodic process that enables the recovery of crossovered CO2 without additional energy input (doi.org/10.1038/s41467-022-30677-x). In this report, an all-liquid anodic process was adopted. CO2 reduction reaction was coupled with glucose oxidation reaction (GOR) instead of OER, offering a low full-cell voltage of 1.9V. CO2 was recovered from anode via facile gas/liquid separation and fed to cathode with fresh CO2, achieving a total carbon efficiency of 48%, and a 46% reduction in energy intensity compared to the state-of-art single stage CO2 to C2+ devices. The strategy is compatible with today’s highest-efficiency electrolyzers and CO2 catalysts that function optimally in neutral and alkaline electrolytes.
Try your own CO2 electrolytic system with Dioxide Materials’ cell hardware and Sustainion® anion exchange membrane.