Dioxide Materials Has Developed CO2 Electrolyzers With Record Performance
Currents up to 500 mA/cm2 at 3 V
Selectivity (Faraday efficiency) above 95%
Only 6 µV/hr voltage increase, equivalent to a 4 year lifetime
Stable performance demonstrated in 6 month run
Can be integrated with an ethanol fermenter to increase ethanol production
Examples of the Performance Seen With Dioxide Materials’ CO2 electrolyzer
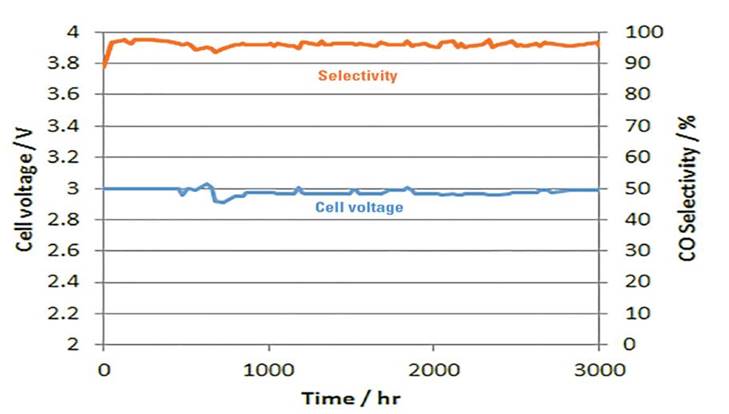
The voltage needed to maintain 200 mA/cm2 at room temperature.
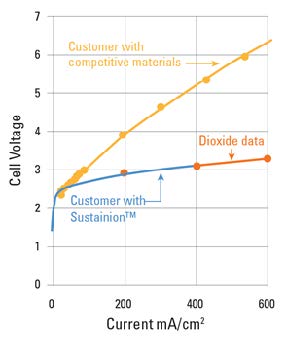
Dioxide Materials’ Sustainion® membranes enable electrolyzers to outperform the competition
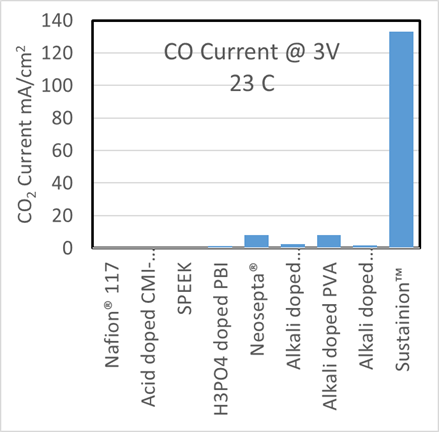
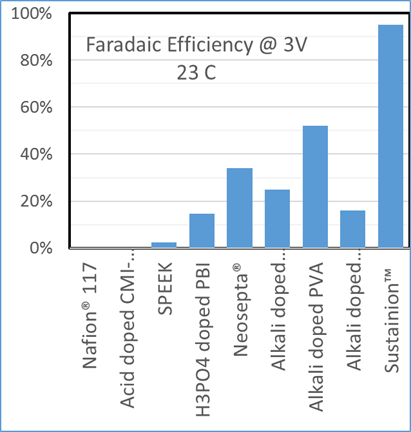
Dioxide Materials’ patented catalysts make the process economic
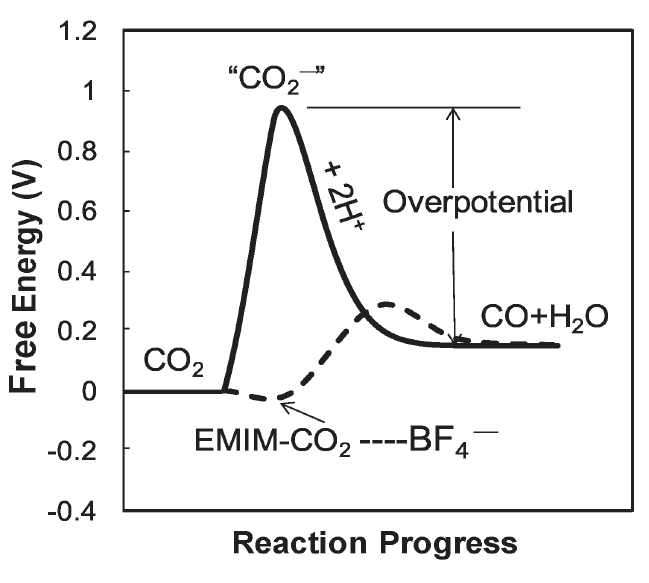
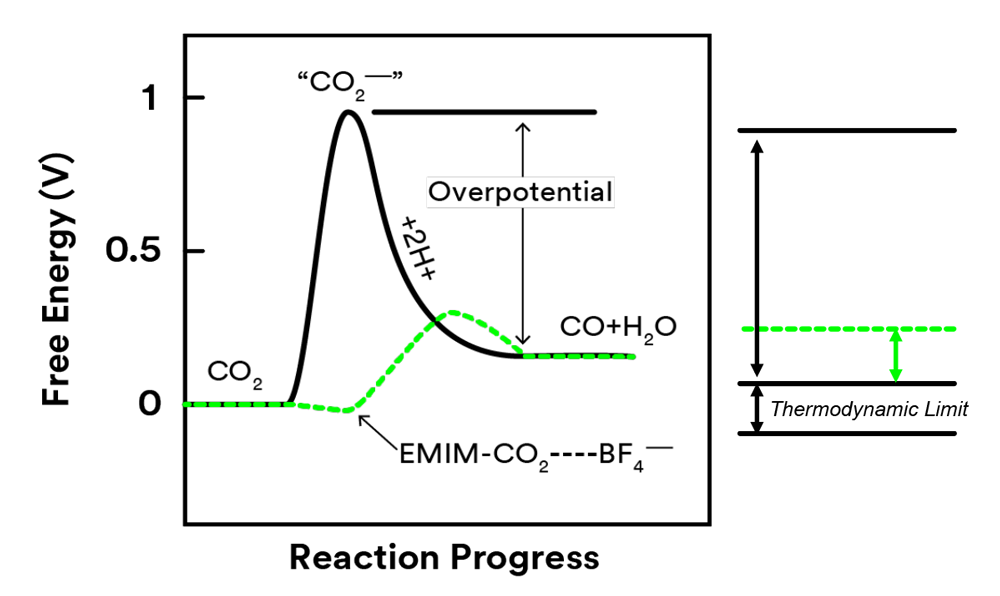
Co-catalyst Lowers the Overpotential
Challenge for electrochemical reduction of CO2
- High overpotential due to the formation of intermediate species
- Low selectivity due to the side reactions
Breakthrough
-
- Combined ionic liquid with Ag metal
- Lowered the overpotential to 0.17V
- Suppressed side reactions
- Improved the selectivity to 98%
Rosen, et al., Science 334, 643 (2011)
Why such good performance?
EMIM creates low barrier pathway

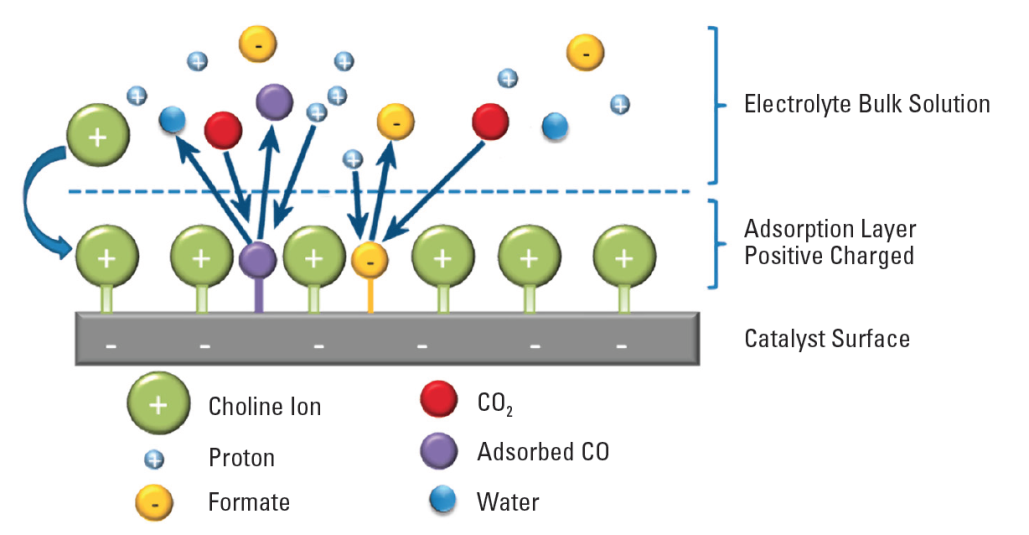
EMIM blocks side reactions
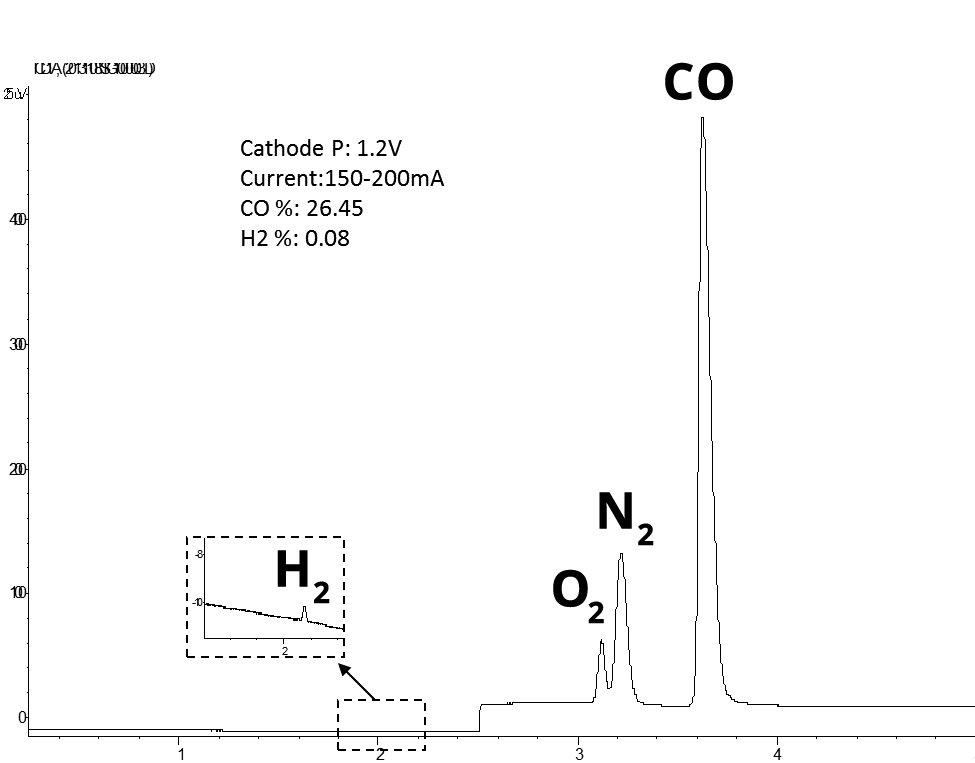
High Selectivity
Dioxide Materials’ Advantages
Better Materials
| Membrane | ASR in 1 M KOH, 60 °C | pH Range</td |
| Sustanion® 37-50 | 0.045 Ω-cm2 | 2-14 |
| Fumasep FAS-50 | 0.37 Ω-cm2 | 0-13 |
| Nafion 115 | 0.52 Ω-cm2 | 0-13 |
| Fumasep FAPQ-375 | 0.83 Ω-cm2 | 0-11 |
| AMI-7001 | 2.0 Ω-cm2 | 0-10 |
| PBI | 8.3 Ω-cm2 | 2-10 |
| Neosepta ACN | >50 Ω-cm2 | 0-8 |
Better Performance


