Developing catalysts with high activity, high selectivity, and long-term stability is an important aspect in electrochemical CO2 reduction (CO2RR). Currently, carbon-based gas diffusion electrodes (GDEs) are the extensively used and investigated electrodes in CO2RR. GDEs reduce mass transport limitations associated with the low solubility of CO2 in aqueous electrodes by providing gaseous CO2 to and near the catalyst. However, carbon-based GDEs have flooding and subsequent degradation issues during long-term operation. To prevent flooding and achieve stable CO2RR, a new class of GDEs based on hydrophobic polymer substrates have been developed and attracted great attentions because of their outstanding CO2RR activity and selectivity. A few different explanations have been proposed to explain their excellent performance, such as the formation of abrupt gas-liquid interfaces, optimized combination of catalyst and ionomer mixture, and advanced electrolyzer design. In a recent work published in ACS Appl. Energy Mater. (https://doi.org/10.1021/acsaem.2c03054), Senocrate and coworkers focused on the properties of the GDE polymer substrate itself and demonstrated that product selectivity, performance stability and resilience to impurities of Ag GDEs are strongly affected by the pore size of the GDE substrate, providing a scalable strategy to improve the CO2RR performance.
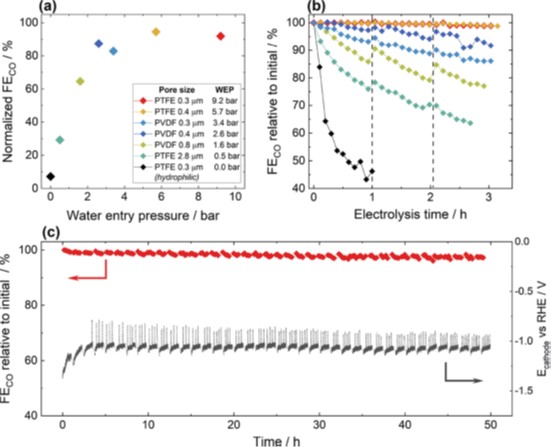
The authors used two sets of commercial polymer fiber substrates, composed of polytetrafluoroethylene (PTFE) or polyvinylidenefluoride (PVDF), with different pore sizes in the range of 0.2 to 3 µm, to fabricate GDEs with a Ag catalyst layer. The GDEs were then analyzed using a three-compartment electrolysis cell. The Faradaic efficiency (FE) of gaseous products (H2, CO) was recorded for performance evaluation. The authors adopted the water entry pressure (WEP) to quantify the GDE substrate’s resistance property to aqueous electrolyte penetration as a function of the pore size and the hydrophobicity of the substrate polymer. The FE and WEP of the GDEs were then correlated to evaluate the effect of substrate pore size and wetting behavior on the CO2RR performance.
The authors observed that GDEs with low WEP show inferior selectivity toward CO (<30% at 100 mA/cm2) as well as low stability (only 65% of initial selectivity toward CO retained after 3 h electrolysis). In stark contrast, GDEs with higher WEPs yield remarkably high selectivity toward CO (up to 95% at 100 mA/cm2) and long-term stability (∼97% of initial selectivity toward CO retained after >40 h electrolysis). This corresponds to a multi-fold improvement of the GDE selectivity and stability obtained solely by acting on the substrate and ensuring a high WEP. The authors also assessed the sensitivity to surface Cu contaminants and find that substrates with high water entry pressures lead to GDEs that are more resilient to impurities, with an almost unvaried selectivity even when contaminated with >1 at % Cu vs Ag.
These results highlight how acting on the substrate is a powerful and scalable way for improving the performance of GDEs for electrochemical CO2 reduction. It also sheds light on identifying prominent failure mechanisms and accelerates future developments of electrochemical CO2 reduction technology.