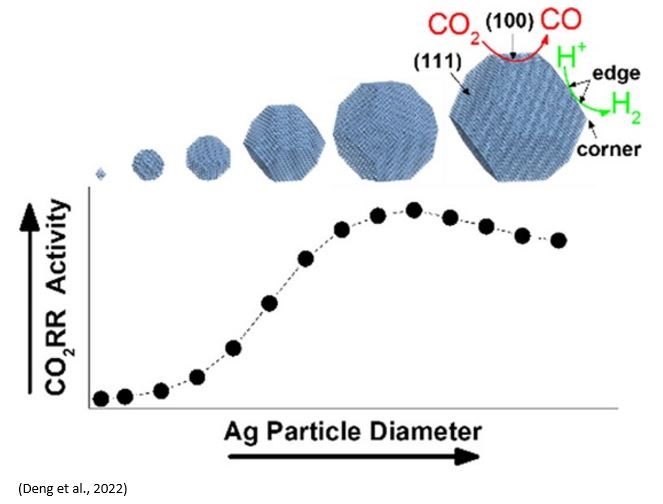
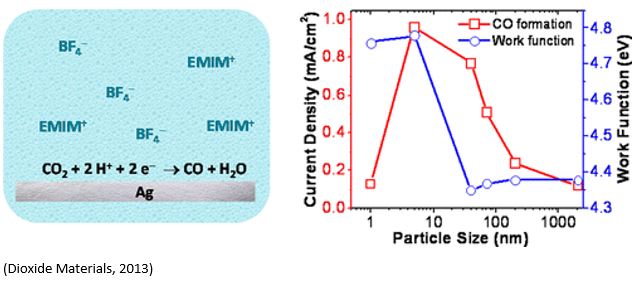
Silver (Ag) is the state-of-the-art electrocatalyst for selective carbon monoxide (CO) production from waste carbon emissions. Deng et al. (https://doi.org/10.1021/acscatal.2c00960) recently investigated the size-dependent transition between CO2 reduction reaction and H2 evolution reaction on silver nanoparticle electrocatalysts. Ag nanoparticles were grown under ultrahigh vacuum (UHV) via metal evaporation onto highly oriented pyrolytic graphite (HOPG) substrate. The results showed that CO2RR rates increased while HER rates decreased when the particle size increased from 2 to 4nm. 3.7 ± 0.7 nm diameter particles demonstrated the highest combination of CO2RR activity and selectivity. This is consistent with the computational modelling of 1-10nm Ag particles with maximum CO2RR activity on 3.7nm Ag particles. Smaller particles favor HER due to a high population of Ag edge sites. CO2RR activity increases for larger particles as the population of Ag(100) surface sites grow. However, a growing population of electrochemically inaccessible, interior atoms eventually decreased catalyst utilization for particle diameters above ∼4 nm. Years ago, Dioxide Materials showed similar size dependent of Ag particle catalysts on the activity of CO2RR( https://doi.org/10.1021/jp310509z).
Electrochemical evaluation of CO2 reduction reaction (CO2RR) was conducted in CO2-saturated 0.1M KHCO3 using an H-cell in Deng’s paper, but in EMIM-BF4 containing 75ppm of water using three-electrode glass cell. Dioxide Materials have improved the current density of CO2RR by two orders of magnitude using gas-feed zero-gap cells where unique Sustainion anion exchange membranes were employed as compared to traditional electrochemical cells used in Deng’s paper (2022) and previous Dioxide Materials’ paper (2013).
Do you want to improve your performance by two orders of magnitude, try Dioxide Materials’ unique Sustainion® membranes and electrochemical hardware.