Cu-based gas diffusion electrodes (GDEs) show excellent performance in the electrochemical reduction of CO2 to ethylene. For the preparation of a GDE, a conductive ionomer is typically mixed with the electrocatalyst in a solvent to prepare an ink, followed by spraying of the mixture onto a gas diffusion layer (GDL) of a carbon cloth or carbon paper. This procedure should result in Cu particles in a homogeneous distribution and a low degree of agglomeration. Generally, ink formulation greatly impacts the catalyst distribution and the degree of agglomeration. However, relatively few studies have investigated the effect of ink composition on the performance of Cu-based GDLs in electrochemical reduction of CO2. In a recent paper published in ACS EST Engg. (ACS EST Engg. 2021, 1, 12, 1649–1658, https://doi.org/10.1021/acsestengg.1c00228), Mul and coworkers investigated how different solvents affect the morphology of Cu/Nafion deposits and how that determines the performance of the obtained catalyst layer (CL).
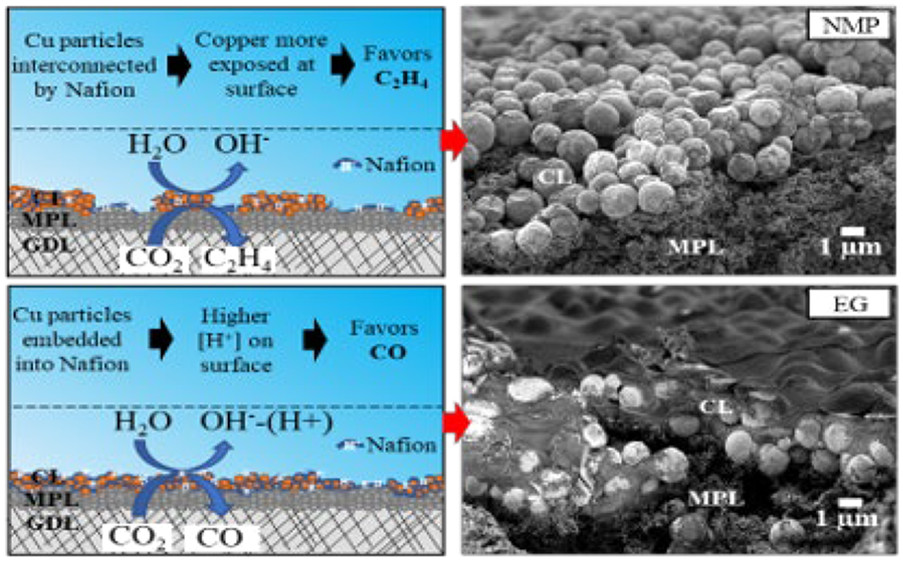
In this work, the authors evaluated how the solvent of ink formulations containing Nafion-ionomer and unsupported Cu particles affects the polymerized Nafion-copper distribution in the as-prepared GDE and the obtained performance in the electrochemical reduction of CO2. Isopropanol (IPA), dimethyl sulfoxide (DMSO), ethylene glycol (EG), and N-methyl-2-pyrrolidone (NMP) were used as solvent for the ink. The GDEs were prepared by spray coating method. The authors observed that DMSO-, EG-, and NMP-based inks consist of similar average Cu particle size and high homogeneity, while IPA-based ink consists larger particle size and some inhomogeneity, indicating a large degree of polymerization of Nafion. The morphology and structure of CL were characterized by different techniques. It was found that the protic solvents (EG, IPA) exhibited less segregation of Nafion and copper particles, leading to a rather homogeneously mixed catalyst-Nafion layer with just a few areas of higher copper or Nafion exposure. The opposite results were obtained with aprotic solvents (DMSO, NMP). As for the surface-exposed copper, the characterization shows the following order: NMP ~ DMSO >> IPA > EG. This is because aprotic solvents prevent Nafion polymerization / agglomerization in the ink before spraying and drying, resulting in an exposed Cu surface.
Electrochemical evaluation results suggested that DMSO and NMP samples exhibited higher values of electrochemical active surface area (ECSA), compared to EG and IPA-based GDEs. When tested in CO2 reduction flow cell, DMSO and NMP-based GDEs provided the best ethylene production performance among all tested GDEs in the presented work (faradaic efficiency: 27.5%). This work clearly points out the importance of controlling ink composition to tune electrochemical properties of the catalyst layer, especially towards the CO2 reduction selectivity to ethylene and other C2 products on Cu-based GDEs, which will certainly benefit the electrochemical CO2 reduction research and general GDE development.